
Molluscum can finally be treated at home or on the go
Because parents have enough to worry about
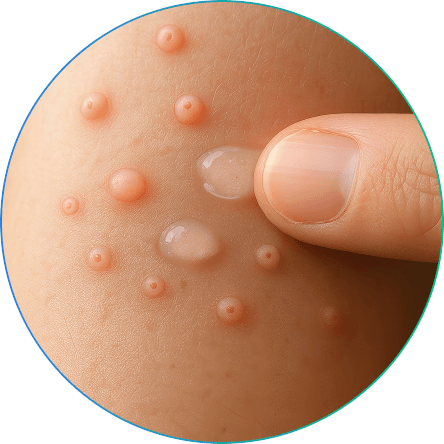
The first and only FDA-approved at-home topical medication for treating molluscum contagiosum in patients 1 year of age and older1-3
FDA=Food and Drug Administration.
Not an actual patient.
ZELSUVMI™ (berdazimer) is a nitric oxide–releasing gel indicated for the topical treatment of molluscum contagiosum in patients 1 year of age and older1,3
With proven efficacy and an established safety profile, ZELSUVMI is the only FDA-approved topical medication for molluscum contagiosum for at-home use1,2
See ZELSUVMI Clinical Trial Results
View the Safety Profile of ZELSUVMI
Learn How ZELSUVMI Is Applied
Help Patients Get Started on ZELSUVMI
Learn More About the Impact of Molluscum in Teens and Adults
INDICATION
ZELSUVMI is a nitric oxide (NO) releasing agent indicated for the topical treatment of molluscum contagiosum in adult and pediatric patients 1 year of age and older.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Application site reactions, including allergic contact dermatitis, have occurred in patients treated with ZELSUVMI. Suspect allergic contact dermatitis in the event of pain, pruritus, swelling, or erythema at the application site lasting longer than 24 hours. If allergic contact dermatitis occurs, discontinue ZELSUVMI and initiate appropriate therapy.
ADVERSE REACTIONS
The most commonly reported adverse reactions (incidence ≥1%) are application site reactions, including pain (such as burning or stinging sensations, 18.7%), erythema (11.7%), pruritus (5.7%), exfoliation (5.0%), dermatitis (4.9%), swelling (3.5%), erosion (1.6%), discoloration (1.5%), vesicles (1.5%), irritation (1.2%), and infection (1.1%). Other adverse reactions include pyrexia (2.2%), vomiting (1.3%), and upper respiratory tract infection (1.2%).
USE IN SPECIFIC POPULATIONS
Pregnancy: There are no available data with use of ZELSUVMI in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
Lactation: There are no data on the presence of berdazimer in human or animal milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ZELSUVMI and potential adverse effects on the breastfed child.
Pediatric: The safety and effectiveness of ZELSUVMI have not been established in pediatric patients younger than 1 year of age.
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. You may request medical information and report adverse events or product complaints to Pelthos Inc. by calling 1-855-330-7546 or sending an email to medinfo@pelthos.com.
Please see the full Prescribing Information and Instructions for Use for ZELSUVMI.
References: 1. ZELSUVMI™ (berdazimer) topical gel, Prescribing Information. LNHC, Inc., Durham, NC; 2024. 2. Keam S. Berdazimer topical gel, 10.3%: first approval. Drugs. 2024;84(3):363-368. 3. Browning JC, Enloe C, Cartwright M, et al. Efficacy and safety of topical nitric oxide-releasing berdazimer gel in patients with molluscum contagiosum: a phase 3 randomized clinical trial. JAMA Dermatol. 2022;158(8):871-878.


